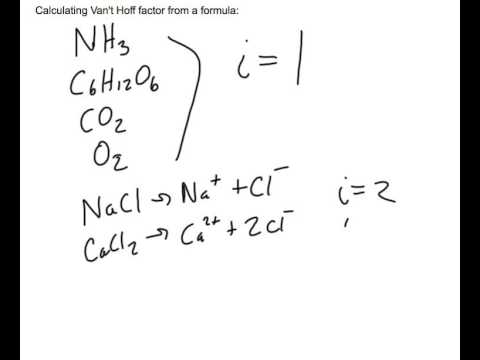
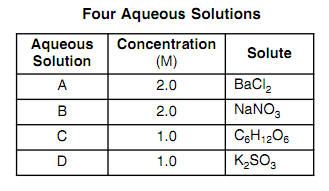
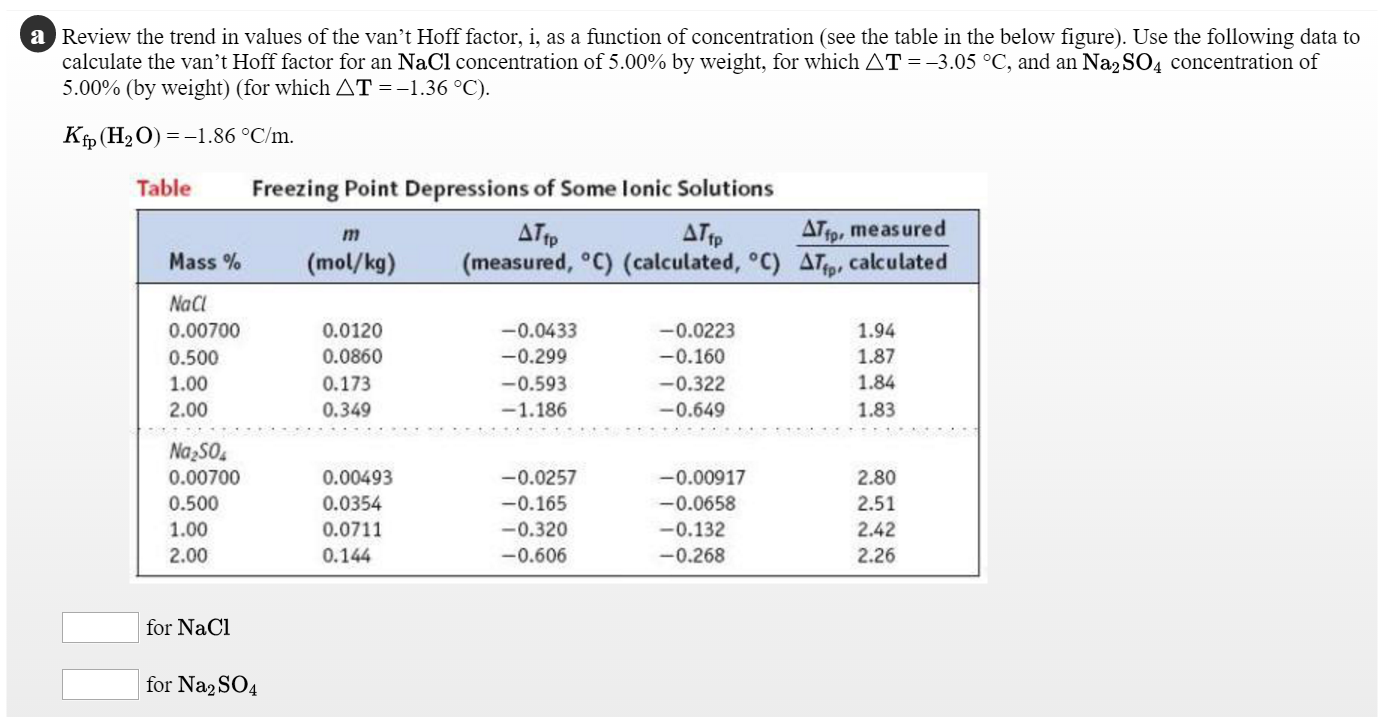
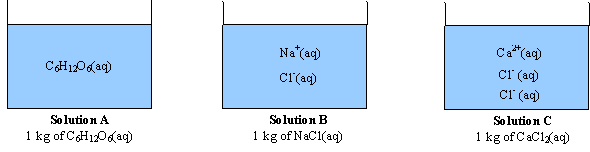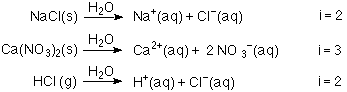What is the van 't Hoff factor for the NaCl in aqueous solution (assuming the complete dissociation of NaCl)? - Quora

Van't Hoff factor as a function of concentration (calculated according... | Download Scientific Diagram
128. Which one has same Van't Hoff factor i as that of Hg2Cl2: (1) NaCl (3) Al(NO3)3 (2) Na2SO4(4) Al,(SO4)3

Welcome to Chem Zipper.com......: The van't Hoff's factor for 0.1 M Ba(NO3)2 solution is 2.74. the degree of dissociation is.