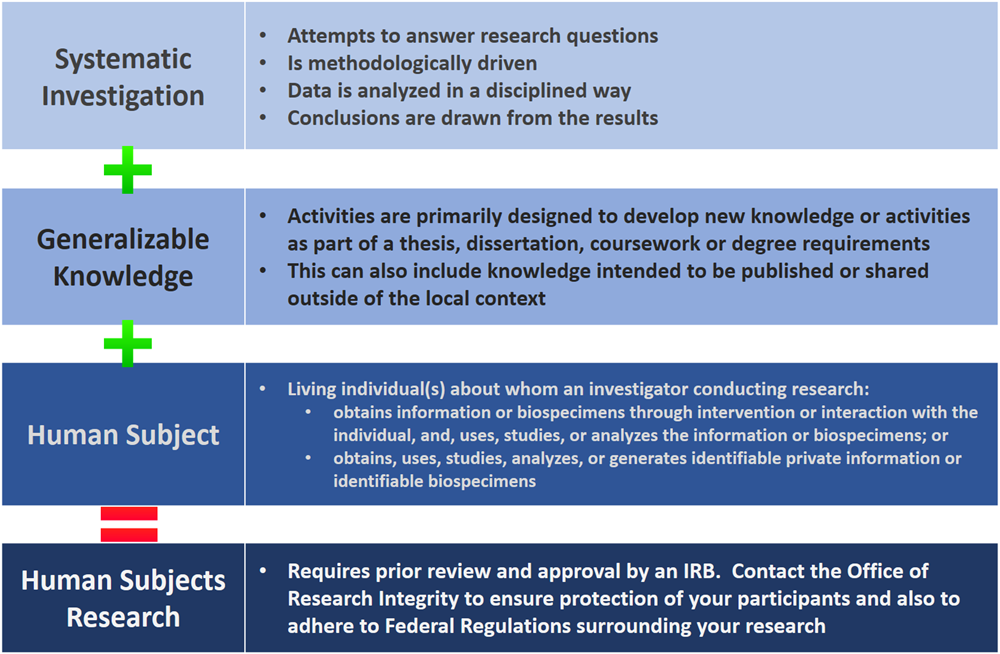
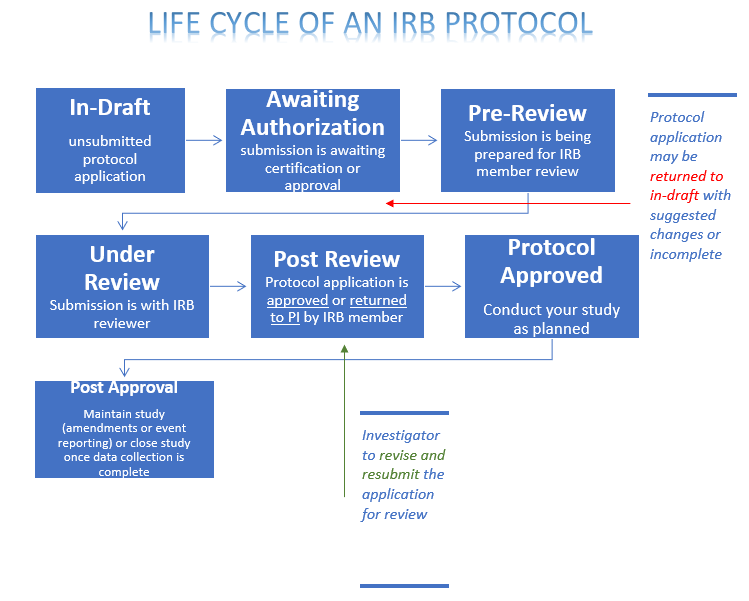
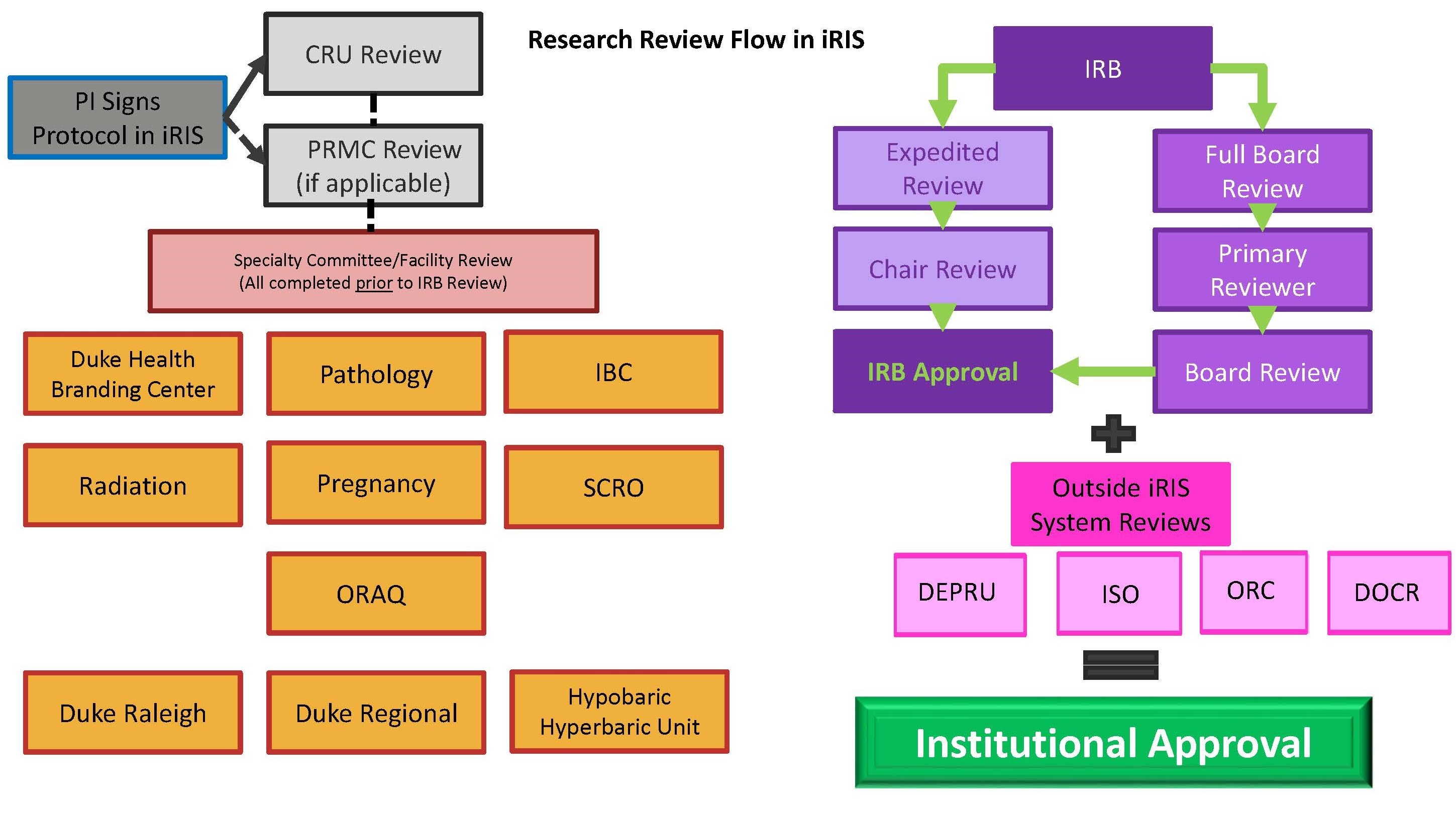
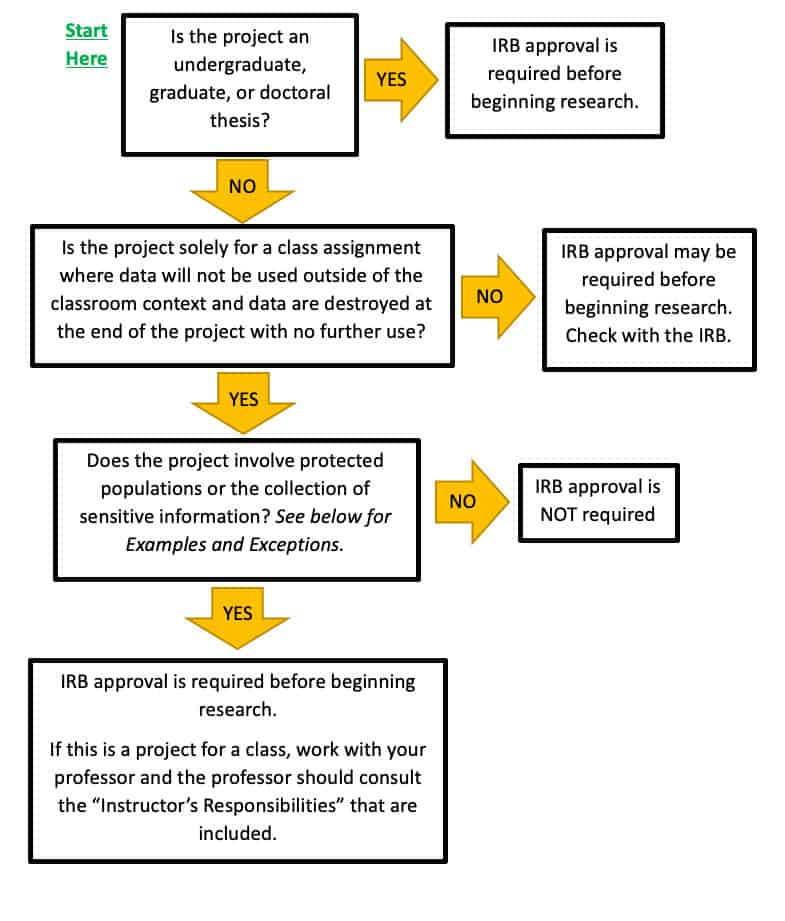
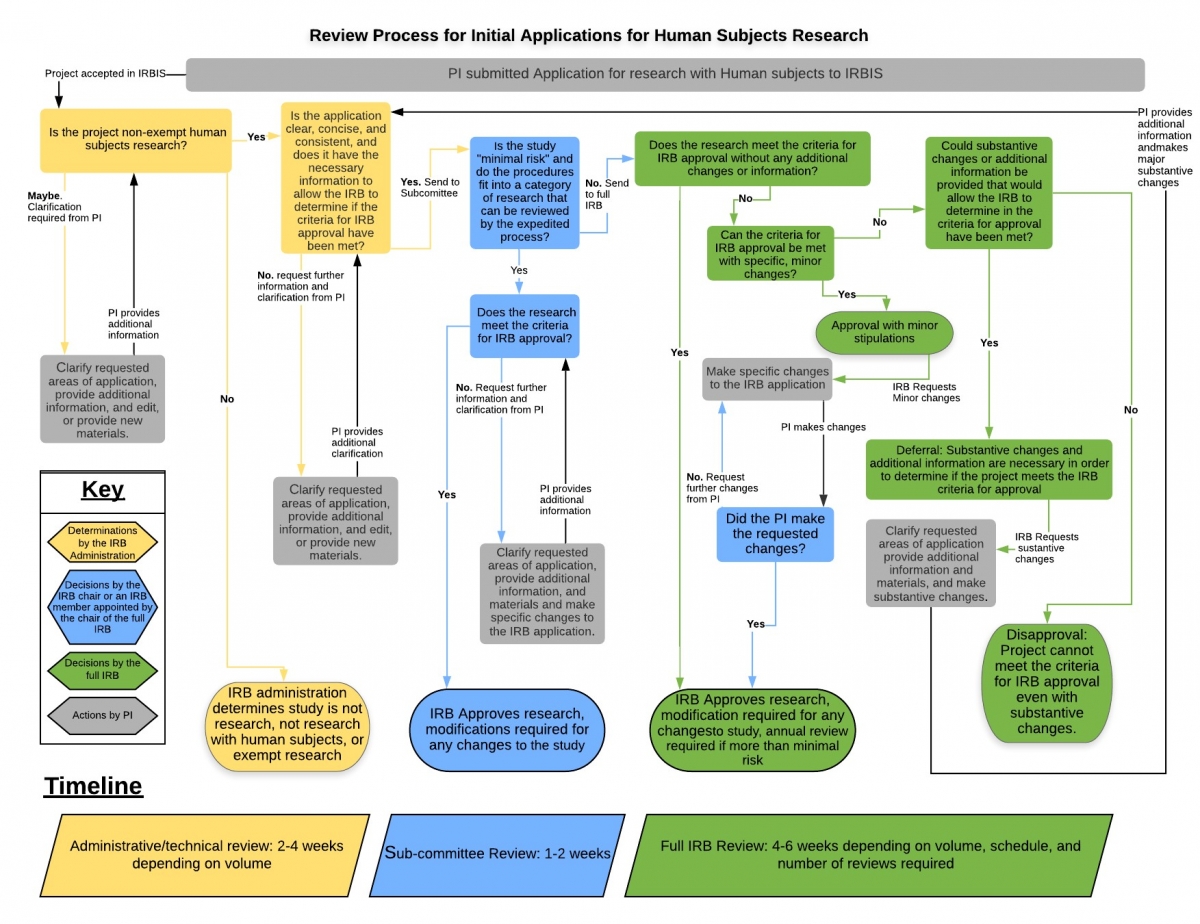
Research Involving Human Subjects All research involving the participation of human subjects must be submitted for review by the IRB (Institutional Review. - ppt download
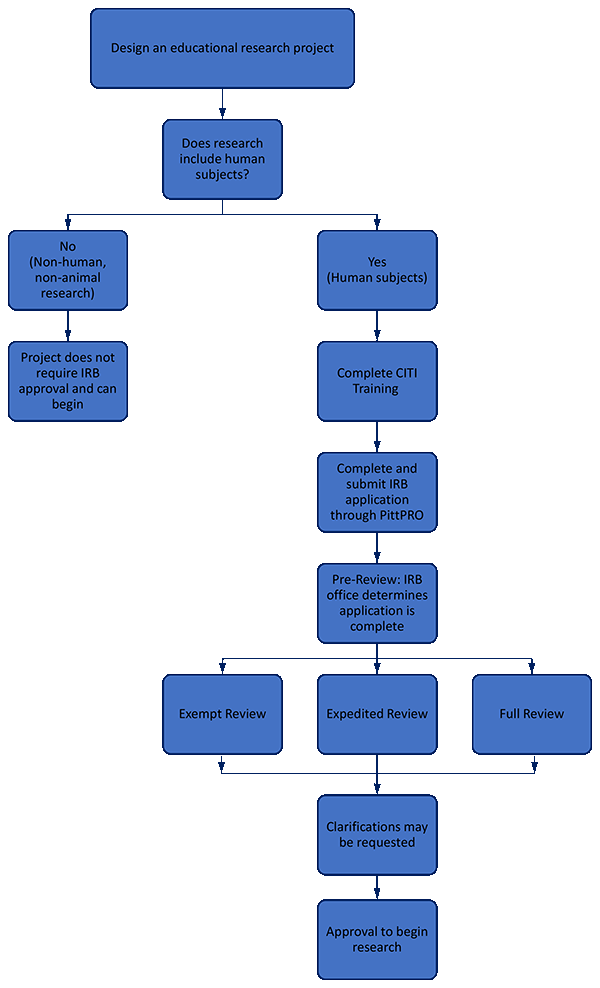
Submitting an IRB application for Educational Research | School of Nursing | University of Pittsburgh
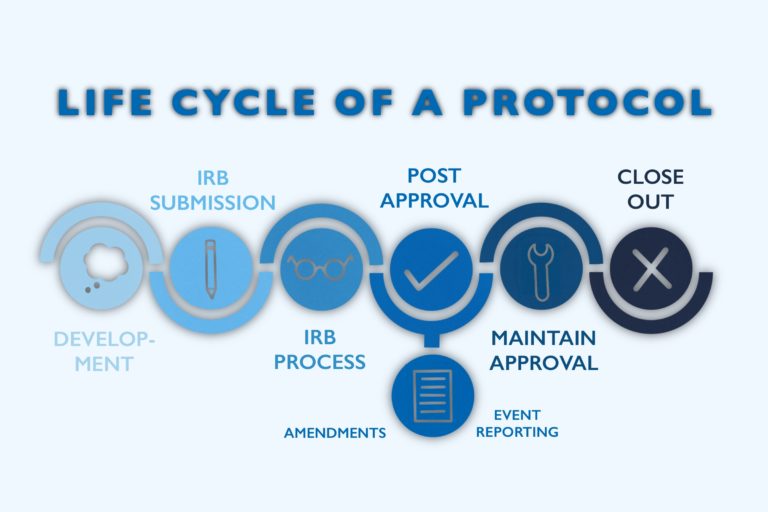
How do Institutional Review Boards (IRB) and Ethics Committees (EC) impact clinical trials? - Clincierge
Master's Students and the IRB | IRB Blog | Institutional Review Board | Teachers College, Columbia University
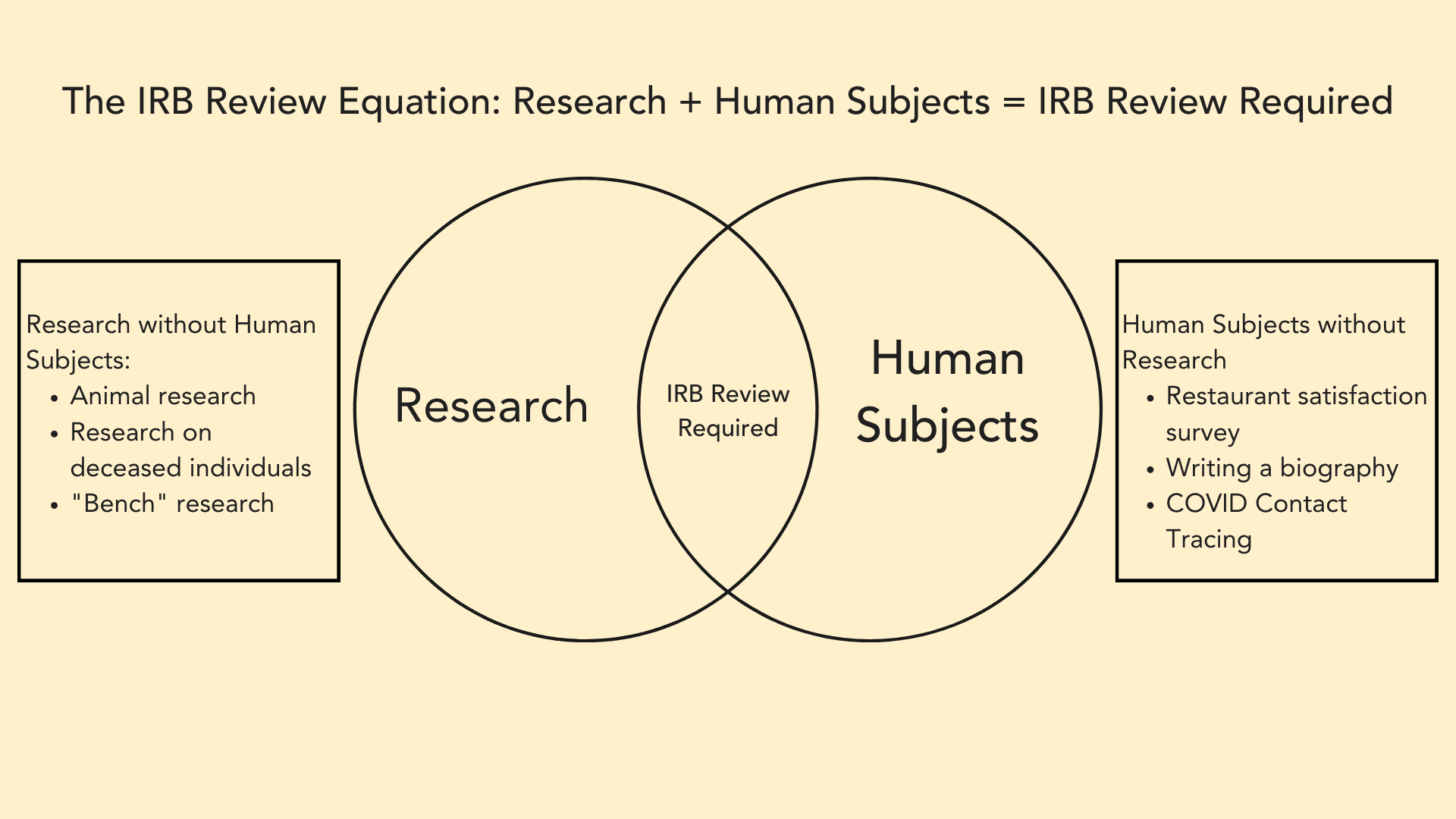
Deep Dives: What is the Difference Between “Exempt” Human Subjects Research, and Projects that are Not Human Subjects Research (NHSR)? – VCU Human Research Protection Program (HRPP) Blog